Data flow in the NNAP
Below is a diagram detailing the updated NNAP data flow. A more detailed diagram of the NNAP data flow is also available in the downloads section below:
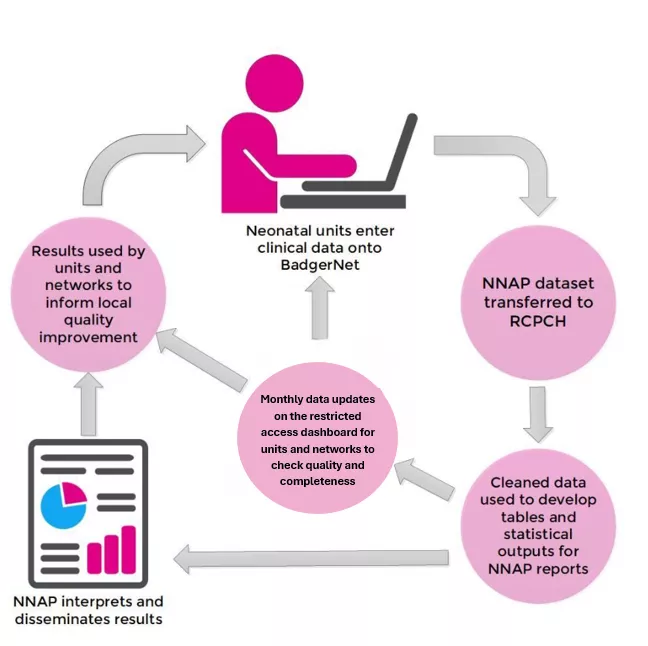
In the Downloads section below you will be able to view copies of the Health Research Authority Confidentiality Advisory Group (CAG) letters of support for the updated NNAP data flow and required amendments.
Privacy and data protection
National Data Opt-Out (England)
The NNAP has been granted a deferral from the NHS National Data Opt-Out (NDO). Full details can be found in the CAG deferral letter from December 2022, available to download below. This means that the National Data Opt-Out should now not be applied to the confidential patient information used without consent for the NNAP. Full details of the NNAP NDO and the associated privacy notices can be found here
You can read more about this on the NHS Digital website, which also has a list of programmes to which the NDO should not be applied.
Please note that parents still have the right to choose for their baby’s information to not be used for the purpose of the NNAP by informing staff on the neonatal unit where they are receiving care. This should be recorded by the neonatal unit within the BadgerNet clinical system using the opt out check box.
Outlier management policy
The NNAP manages outliers according to the NNAP: Detection and management of outlier status for 2024 data policy and in line with HQIP guidance for the detection and management of outliers in England and Wales.
Choice of performance indicators for 2024 data outlier analysis
The performance indicators subject to outlier analysis are selected by the NNAP Methodology and Dataset Group and endorsed by the NNAP Project Board.
For the 2024 data year, the NNAP conducts low outlier analysis on the following measures at unit level:
- Is a mother who delivers a baby below 30 weeks gestational age given magnesium sulphate in the 24 hours prior to delivery?
- Does a baby born at less than 34 weeks gestational age have their cord clamped at or after one minute?
- Does an admitted baby born at less than 32 weeks gestational age have its first measured temperature of 36.5°C to 37.5°C within one hour of birth?
- Does a baby born at less than 30 weeks gestational age receive medical follow-up at two years gestationally corrected age (18-30 months gestationally corrected age range of acceptable ages)?
- Does an admitted baby born at less than 32 weeks gestational age meet the NNAP surveillance definition for necrotising enterocolitis (NEC) on one or more occasion?
- Does an admitted baby have one or more episodes of bloodstream infection, characterised by one or more positive blood cultures taken, after 72 hours of age?
- Does an admitted baby born at less than 32 weeks’ gestational age develop bronchopulmonary dysplasia (BPD) or die?
- Does a baby born at less than 32 weeks’ gestational age have a complete intraventricular haemorrhage scan within 28 days of birth? (Missing data outlier)
- Does a baby born at less than 31 weeks gestational age, or weighing less than 1501g at birth undergo the first ROP screening according to the guideline?
- Does a baby born at less than 34 weeks gestational age receive any of their own mother’s milk in the first two days of life?
- Does a baby born at less than 32 weeks gestational age only receive non-invasive breathing support* during the first week of life?
For the 2024 data year, the NNAP will notify high outliers for the following measures:
- Does an admitted baby born at less than 32 weeks gestational age have its first measured temperature of 36.5°C to 37.5°C within one hour of birth?
- Is a mother who delivers a baby below 30 weeks gestational age given magnesium sulphate in the 24 hours prior to delivery?
- Does a baby born at less than 30 weeks gestational age receive medical follow-up at two years gestationally corrected age (18-30 months gestationally corrected age range of acceptable ages)?
- Does a baby born at less than 31 weeks gestational age, or weighing less than 1501g at birth undergo the first ROP screening according to the guideline?
- Does an admitted baby born at less than 32 weeks’ gestational age develop bronchopulmonary dysplasia (BPD) or die?
- Does a baby born at less than 34 weeks gestational age have their cord clamped at or after one minute?
- Does a baby born at less than 34 weeks gestational age receive any of their own mother’s milk in the first two days of life?
Full details can be found below in the NNAP outlier management policy for 2024 data
Trust quality account information
The NNAP is included in the NHS England Quality Accounts list, which is available from the Healthcare Quality Improvement Partnership (HQIP) website.
HQIP also publishes the National Clinical Audit and Enquiries Directory which should answer most of your questions relating to the NNAP.
All neonatal units in England, Wales and Scotland are eligible to participate in the NNAP. The audit expects 100% case ascertainment from participating units. To find the list of participating units for the latest published year of results, see NNAP extended analysis on 2023 data report.
The table below summarises NNAP data collection periods and expected report publication dates.
| Data collection period | Expected annual report publication date |
|---|---|
| 1 January 2024 to 31 December 2024 | October 2025 |
| 1 January 2025 to 31 December 2025 | October 2026 |
Data dictionary
You can view the full NNAP data dictionary for the 2024 data year on our NNAP measures page.
Methodology and Statistical Analysis Plan
The purpose of this document is to provide a detailed methodological overview of the data and measure development steps and analyses contained in the NNAP annual reports. It is not designed to relate specifically to any one report year but will be periodically updated annually to ensure that it is in line with the latest NNAP methodology. You can download the NNAP Methodology and Statistical Analysis below.









