The recommendations within this guidance are based on existing principles of management of acute hepatitis and acute liver failure and on expert consensus opinion in the absence of high quality evidence around this novel condition.
This clinical framework has been produced using a modified Delphi consensus process and will be reviewed by Professor Simon Kenny and Professor Rachel Harwood.
Introduction
An increase in the frequency of non-A-E hepatitis in children has been noted in the UK and internationally since the beginning of January 2022. The UK Health and Security Agency (UKHSA) is investigating the aetiology of this increase in cases and provides case definitions as follows.1
|
Confirmed: A person presenting since 1 January 2022 with an acute hepatitis which is not due to hepatitis A-E viruses, or an expected presentation of metabolic, inherited or genetic, congenital or mechanical cause with serum transaminase greater than 500 IU/L, who is 10 years old and under. Possible: As above in a child aged 11-15 years old |
The working hypotheses for the aetiology of this remains under review. Currently the leading hypotheses involve adenovirus but alternative causes including SARS-CoV-2 and toxicological causes remain under review.1
This clinical framework is intended to provide a structure for the investigation, transfer and management of children with novel non-A-E acute hepatitis in the context of an emergence of a novel disease. Whilst the framework provides general principles of care for this condition, each case should be treated in an individual and nuanced way. The recommendations within the guidance are based on existing principles of management of acute hepatitis and acute liver failure and on expert consensus opinion in the absence of high quality evidence around this novel condition. Details of the methodology are available in appendix 1.
Definitions
Taken from BSPGHAN guidelines (PDF)
Liver disease with acute onset in children aged over 3 months:
Either
- sudden onset of jaundice with evidence of liver aetiology, or
- incidental discovery of raised transaminases in association with symptoms suggesting acute onset.
Acute liver failure:
Either
- INR >2.0 due to liver dysfunction of less than 8 weeks duration without encephalopathy, or
- INR >1.5 with encephalopathy.
Clinical suspicion
- Novel non-A-E acute hepatitis should be considered in children with a clinical picture of hepatitis and elevated transaminases after exclusion of A-E hepatitis.
- Notification - All cases of novel non-A-E acute hepatitis should be notified (in office hours) to the local health protection team for public health follow-up:
Initial investigations
- Initial investigations should include full blood count, urea, creatinine, electrolytes, calcium, magnesium, phosphate, liver function tests (total and conjugated bilirubin, ALT, AST, Alk Phos, GGT), albumin, coagulation studies including INR, blood culture, ammonia, blood sugar, venous blood gas, urine culture, ferritin, alpha fetoprotein and SARS-CoV-2 PCR. Paracetamol levels should be checked if age and history appropriate.
- Abdominal ultrasound including the liver and doppler evaluation of the hepatic vessels should be performed in the local hospital.
- Testing for immunoglobulins, autoantibodies, pANCA and caeruloplasmin if age appropriate as per BSPGHAN guidance.
- If Hepatitis A-E is excluded then consider testing for other infections which may be associated with acute hepatitis in children with a serum transaminase >500 IU/L and in children with lower transaminase levels with clinical concern. Testing should be directed by clinical presentation and exposure history.
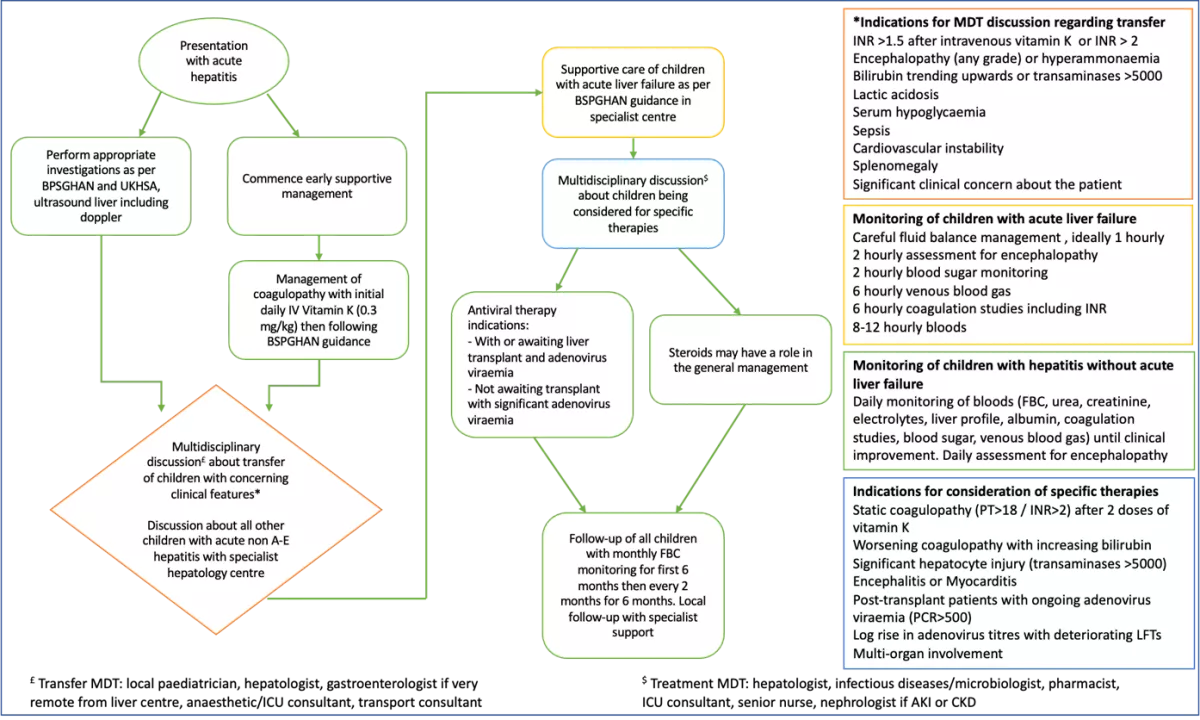
Discussion and transfer to specialist centre
- All children with acute non-A-E hepatitis should be discussed with a specialist hepatology centre.
- Urgent discussion with a specialist hepatology team should be prompted by any of the following clinical features:
- INR >1.5 after intravenous vitamin K
- INR > 2
- Encephalopathy (any grade)
- Bilirubin trending upwards
- Lactic acidosis
- Serum hypoglycaemia
- Transaminases >5000
- Significant clinical concern about the patient
- Sepsis
- Cardiovascular instability
- Hyperammonaemia
- Splenomegaly.
- Multidisciplinary discussion about children with any of the above clinical features is recommended if the child requires transfer to a specialist centre.
- The multidisciplinary team should include:
- Local consultant paediatrician
- Consultant hepatologist
- Consultant gastroenterologist in tertiary centre if very remote from specialist liver centre
- An anaesthetic / ICU consultant
- A paediatric transport consultant.
Monitoring
- Children with acute liver failure: whilst awaiting urgent transfer to specialist liver centre and within specialist centre
- Careful fluid balance management, ideally 1 hourly fluid balance
- 2 hourly assessment for encephalopathy
- 2 hourly blood sugar monitoring
- 6 hourly venous blood gas
- 6 hourly coagulation studies including INR
- 8-12 hourly bloods (FBC, urea, creatinine, electrolytes, liver profile, albumin).
- Acute hepatitis without acute liver failure
- Daily monitoring of bloods (FBC, urea, creatinine, electrolytes, liver profile, albumin, coagulation studies, blood sugar, venous blood gas)
- Daily assessment for encephalopathy
- Blood test monitoring can reduce in frequency
- For immunocompetent children adenovirus PCR measurement (blood) can be stopped after two negative tests a few days apart or, in positive patients, when the clinical status is improving and viral load reducing after discussion with the infectious diseases or microbiology or virology specialists within the Paediatric Liver Centre
- When there is clinical and biochemical improvement
- When there is normalisation of coagulation (INR<1.3, PT<14)
Management
- Vascular access:
- Central IV access should be placed in the specialist centre (Consensus phase 1, 70% strongly agree) and should be considered early for children meeting criteria for transfer.
- Supportive care of children with acute liver failure should follow the acute liver failure BSPGHAN guidance (PDF).
- Coagulopathy:
- Children with acute hepatitis and a deranged coagulation profile should initially receive daily IV vitamin K (0.3mg/kg up to 10mg).
- Further management of coagulopathy should follow BSPGHAN guidance in consultation with specialist unit.
- Coagulopathy:
- Specific therapy for novel non-A-E acute hepatitis (ie antiviral and immunosuppressive therapies)
- Children being considered for specific therapy should be discussed by a multidisciplinary team and specific therapy should only be administered after discussion with specialist hepatology centres.
- Members of the multidisciplinary team should include:
- Consultant hepatologist
- Infectious diseases / microbiology consultant
- Pharmacist
- ICU consultant (if the child is on ICU)
- Senior nurse
- Specific therapy for acute non-A-E hepatitis should be considered in children with:
- Static coagulopathy (PT>18 / INR>2) after 2 doses of vitamin K
- Worsening coagulopathy with increasing bilirubin
- Significant hepatocyte injury (ALT >5000 IU/L)
- Encephalitis
- Myocarditis
- Post-transplant patients with ongoing adenovirus viraemia (PCR>500)
- Log rise in adenovirus titres in combination with deteriorating liver function tests
- Multi-organ involvement
- Antiviral therapies
- In CYP without liver transplant and not currently awaiting liver transplant, meeting one of the above criteria for consideration of specific therapies, antivirals should be considered in patients with:
- proven significant adenovirus viraemia (blood, PCR)
- In CYP with liver transplant or awaiting liver transplant antiviral therapies should be considered in patients with adenovirus viraemia
- Children being considered for antiviral therapies should have other infective causes of hepatitis definitively excluded prior to starting treatment
- Cidofovir is currently the antiviral of choice
- If Brincidofovir became available this would be the antiviral therapy of choice
- Intravenous pre-hydration should be given before Cidofovir in children without acute liver failure
- Oral Probenecid should be given concurrently with Cidofovir
- Children with normal renal function should receive 5mg/kg Cidofovir initially once per week
- Children with abnormal renal function (creatinine clearance <0.3ml/min/kg) and acute non-A-E hepatitis should be discussed with a pharmacist before commencing Cidofovir
- Children with abnormal renal function (creatinine clearance <0.3ml/min/kg) and acute non-A-E hepatitis should be discussed with a nephrologist before commencing Cidofovir
- Children receiving Cidofovir should have careful fluid balance monitoring Renal function should be measured before and after administration of Cidofovir
- In CYP without liver transplant and not currently awaiting liver transplant, meeting one of the above criteria for consideration of specific therapies, antivirals should be considered in patients with:
- Immunosuppression
- There may be a role for steroids in the general management of acute non-A-E hepatitis
Follow-up
- Children with novel non-A-E acute hepatitis should undergo routine follow-up for a minimum of 12 months which can be performed or supported by the local paediatric unit.
- Specialist follow-up may be performed remotely in conjunction with local units.
- FBC monitoring should occur monthly for the first 6 months and every other month for the subsequent 6 months as hepatitis associated aplastic anaemia (HAAA) has been reported in seronegative hepatitis. Individuals with evidence of HAAA should be referred to a haematologist. Urea, electrolytes and liver function tests should also be performed.
Research
- As there is much uncertainty around the aetiology and pathogenesis of this disease, colleagues are encouraged to enrol these children to the ISARIC Clinical Characterisation Protocol (IRAS126600, CPMS14152)
- In CYP with novel non-A-E acute hepatitis it would be appropriate to:
- Investigate the role of steroids
- Compare antiviral to no antiviral therapy.
- The BPSU have started collecting data on acute hepatitis in children as a response to the increasing number of cases. This surveillance study aims to understand viral, non-viral or unidentified causes.
Flow diagram of recommended framework of care
Appendix 1
Methods
This process was undertake as a modification of the Delphi consensus process. Statements were scored 1-9 where 1-3 indicates the responder disagrees with the statement, 4-6 indicates that they agree and 7-9 indicates that they strongly agree. Consensus was deemed as being gained when >70% experts strongly agreed with the statement and <15% disagreed.
The first consensus round used Microsoft Forms for responders to score the statements with initial discussion about each statement within a virtual meeting and then independent scoring of each of the statements. When statements did not reach consensus they were discussed individually and reworded if this was felt to be appropriate.
A second consensus round was undertaken using mentimeter with the same scoring system and statements meeting consensus were included in the framework. Statements that did not meet consensus but where was a strong subsequent discussion with recommended wording from experts the statement was included for consideration during wider circulation of this document.
The guidance was then reviewed by a paediatric consultant to ensure that the content was appropriate and accessible for clinicians in non-hepatology centres.
Participants
Phase 1: 17 Delphi participants, 6 consultant hepatologists (4 paediatric, 2 adult), 3 consultant paediatric gastroenterologists, 8 paediatric infectious diseases/microbiology
Phase 2: 20 Delphi participants, 10 consultant hepatologists (8 paediatric, 2 adult) 5 consultant paediatric gastroenterologists, 5 infectious diseases/microbiology
Updates
- 16 June 2022 - Under Research, noted BPSU's surveillance study
- 6 June 2022 - Under Clinical suspicion, added contact details by nation for notification to local health protection team for public health follow-up









