Introduction
The BPSU system allows researchers to access over 3,800 paediatricians. This provides a coordinated data collection system that minimises the reporting burden on clinicians. The BPSU Scientific Committee also provides expertise and support to prospective researchers to ensure that research methodologies are effective and appropriate.
For researchers wishing to have a study included on BPSU 'Orange' eCard reporting scheme they must apply to the BPSU Scientific Committee (SC). As the success of the BPSU methodology relies entirely on the willingness of consultant paediatricians to complete and return the monthly Orange eCard and study questionnaires, it is essential that BPSU studies are scientifically robust, adequately resourced and contribute to clinical and public health practice without putting too great a burden on reporting doctors.
The BPSU is committed to ensuring that patient and public involvement (PPI) is embedded in its work in every way possible. The Unit works with investigators to ensure that public and patient involvement is appropriately considered in any submission considered by the BPSU. Useful resources for public and patient involvement can be found on our patients and the public pages.
The application process has been developed to reflect these responsibilities.
For further information on the BPSU application process please contact BPSU@rcpch.ac.uk.
Eligibility and funding
Studies considered eligible to be undertaken through the BPSU are those in which:
- the condition is a relatively rare childhood disorder or a rare complication of a more common disease of such low incidence or prevalence as to require ascertainment of cases on a national scale. In practice, the condition studied should have an expected incidence in the UK of normally no more than 360 cases per year
- the majority of cases are expected to be seen by seen by paediatricians
- cases can be easily identified and defined using a clear case definition
- study data is easily accessible from the normal clinical notes
- approval to collect unconsented identifiable data is sought from the CAG of the HRA and PBPP.
Examples of studies which would not normally be eligible for study through the BPSU are those which:
- are interventional studies
- require controls
- can be undertaken through a regional study
- can be undertaken though a study involving specialist clinicians only
- require retrospective reporting
- involve any additional clinical intervention for reported cases (other than the results of diagnostic tests on samples collected during routine clinical management).
Application process
There is a two-stage peer review application procedure. Phase one (P1) is an outline application to establish if the study meets the BPSU criteria. Applications should be submitted on the phase one application form. If the phase one application is accepted by the SC, a more detailed phase two application will be requested.
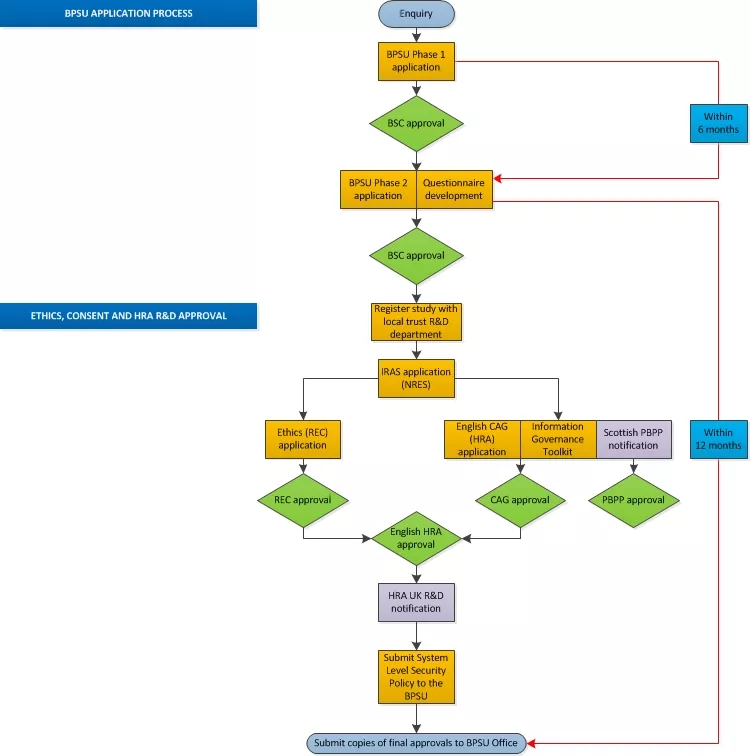
A full outline of the application process is detailed in the image below.
Phase 1
Phase 1 is the submission of an initial application to the BPSU Scientific Committee. If the phase 1 application is accepted by the SC, a more detailed phase 2 (P2) application will be invited.
Note: The SC expects the submission of a phase 2 application within six months of phase 1 approval. If a phase 2 application is not forthcoming, the BPSU Office will contact the principal investigator to discuss removing the submission from the application process. We will give the principal investigator the opportunity to make a formal phase two application before any further action is taken.
Download the phase 1 application guidance and form at the bottom of this page.
Phase 2
If the phase 1 application is approved by the committee, a more detailed phase 2 application should be completed.
Alongside a completed phase 2 application form, the BPSU Scientific Committee (SC) asks that you supply the following:
- signed covering letter from the main contact/principal investigator for the study, which addresses any points raised by the SC in its phase one response letter
- letters of support that you consider relevant for the SC to consider, for example award letters from funding bodies or letters confirming support by collaborating partners such as paediatric specialty groups
- questionnaires, proformas and covering letters that will be used within the study - please provide a version number and date for each
- data analysis plan, with a breakdown of how the questions are to be analysed and how they will address the objectives of the study - examples are available on request
- public information materials, such as a leaflet
- letters of support from supporting organisations, if appropriate.
Download the phase two application guidance and form, and all templates, at the bottom of this page.
Post phase 2
If the phase 2 application is approved, REC (Research Ethics Committee) and CAG-HRA (Confidentiality Advisory Group - Health Research Authority), PBPP (Public Benefit and Privacy Panel) approval should be obtained and the IG (Information Governance) toolkit completed.
Note: Once a phase 2 application has been accepted, the SC expects the study to commence surveillance within 12 months of the Chair’s approval. If surveillance has not commenced witin 12 months of the Chair’s approval, the BPSU office will contact the principal investigator to discuss the application and the reasons for the delay. If a satisfactory response is not made, the BPSU reserves the right to revoke its phase two approval. The study team will be invited to ‘re-submit’ a phase 1 application.
Contributions
The current contributions for running a study through the BPSU is £15,000 for the first year (13 months) and £10,000 a year for subsequent years1.
The charge includes support in completing ethics forms and associated issues and the cost of producing the protocol card (about £500). Further costs may be applicable for additional printing or work.
Application submission dates
Phase one and two applications need to be submitted five weeks before a BPSU Scientific Committee meeting but we may accept applications if they come within three weeks of a meeting. The dates of upcoming SC meetings are listed below:
| Meeting dates | Submission deadline |
| 29 January 2025 | 16 December 2024 |
| 20 March 2025 | 03 February 2025 |
| 15 May 2025 | 06 February 2023 |
| 11 May 2023 | 07 April 2025 |
| 23 July 2025 | 09 June 2025 |
| 18 September 2025 | 04 August 2025 |
| 12 November 2025 | 06 October 2025 |
- 1Please note that for those studies supported by commercial organisation, those applications will be charged at a rate equivalent to the full economic cost of running the study.